Product packaging is an integral part of any industry. When it comes to pharmaceutical packaging, it is possibly even more critical than for other sectors. Pharmaceutical packaging protects the medication or medical device from physical damage, contamination, and external factors affecting the product's efficacy and stability. Proper packaging also ensures the safety of healthcare professionals and patients by providing tamper-evident features.
The pharmaceutical industry is one of the most heavily regulated. These regulations apply to production, all levels of packaging, storage conditions, and other areas. Custom boxes for pharmaceutical products allow pharmaceutical companies to design packaging specifically for their products, ensuring optimal fit and protection. Custom packaging can also be designed to optimize space during transportation.

Pharmaceutical Packaging: FDA, ISO, and PDA Guidelines
The FDA establishes guidelines and regulations that pharmaceutical companies must adhere to throughout drug development and approval. It also regulates drug packaging, labeling and advertising to ensure that the information is accurate and not misleading. Please note that regulations and guidelines may change over time, so referring to the FDA's official website for the most up-to-date information is essential.
ISO (International Organization for Standardization) standards play a crucial role in ensuring the quality, safety, and effectiveness of pharmaceutical packaging. These standards provide guidelines and requirements for various aspects of pharmaceutical packaging processes and materials (ISO 15378:2017, ISO/TS 16775:2021, ISO 11607-1:2019, and others).

The Parenteral Drug Association (PDA) is a global association that provides scientific, technical, and regulatory information and education to the pharmaceutical and biopharmaceutical community. PDA has developed several guidelines and standards to ensure pharmaceutical products' quality, safety, and efficacy.
Product Protection: Ensuring safety and integrity
Packaging is crucial in protecting pharmaceutical products from various external factors. Here are some critical aspects of ensuring the safety and integrity of pharmaceutical products with packaging:
Physical Protection - Packaging is a barrier against physical damage during transportation and handling. Properly designed packaging helps prevent breakage, leakage, or other harm compromising the product's quality and safety.
Light Protection - Some pharmaceutical products are sensitive to light and can undergo degradation or chemical changes when exposed to light. Pharmaceutical packaging should be designed to block or minimize light exposure.
Contamination Prevention - Packaging materials must be chosen carefully to avoid contamination of the pharmaceutical product.
Tamper-Evidence - Tamper-evident medical packaging is crucial to ensure the integrity of products. It shows signs of tampering or opening attempts, alerting patients and healthcare professionals to the potential risks of using compromised products.
Child-Resistant Packaging - Certain pharmaceutical products should be packaged in child-resistant containers to reduce the risk of accidental ingestion by children.
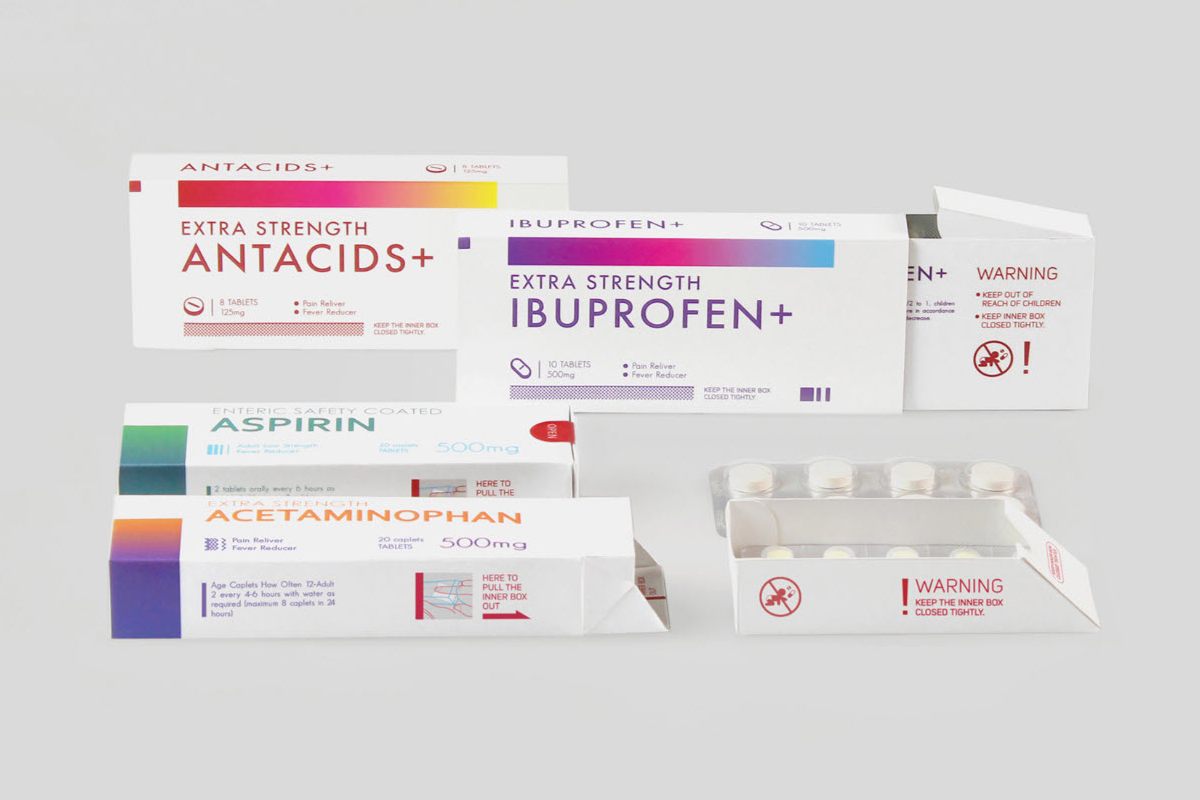
Packaging Materials: Choosing the right fit for pharmaceutical products
A suitable packaging material should protect the pharmaceutical product from external factors while maintaining its chemical and physical properties. Here are some factors to consider when choosing a suitable material for pharmaceutical product packaging:
Product Compatibility - The packaging material must be compatible with the specific pharmaceutical product it will contain, ensuring that the product does not interact with the packaging material.
Barrier Properties - Different pharmaceutical products have varying sensitivities to moisture, oxygen, light, and other environmental elements. The packaging material should provide adequate barrier properties to protect the product.
Physical Protection - Packaging should withstand transportation, handling, and storage without compromising the integrity of the product.
Stability - The packaging material should maintain its structural integrity throughout the product's shelf life.
Regulatory Compliance - Packaging materials must comply with relevant regulations and guidelines set by health authorities, such as the FDA or PDA.
Environmental Impact - Consideration should be given to the ecological impact of the packaging material. Biodegradable or recyclable materials may be preferred to reduce the overall environmental footprint.
The most often used materials for primary packaging are glass, plastic, aluminum (blister packs), films, and laminates.
Common packaging material for secondary packaging is paperboard. It provides protection and information for multiple unit packages.

Choosing the suitable material for pharmaceutical product packaging requires a comprehensive understanding of the product's characteristics, the manufacturing process, and regulatory requirements. Collaboration between packaging experts and pharmaceutical manufacturers is essential.
Suggested Personalized Options: Elevating Pharmaceutical
Product Presentation
While eye-catching displays can enhance marketing efforts, it is important to ensure that custom boxes for pharmaceutical products still meet safety and labeling standards. Collaborating with packaging experts and considering consumer feedback can help optimize the design for maximum impact.
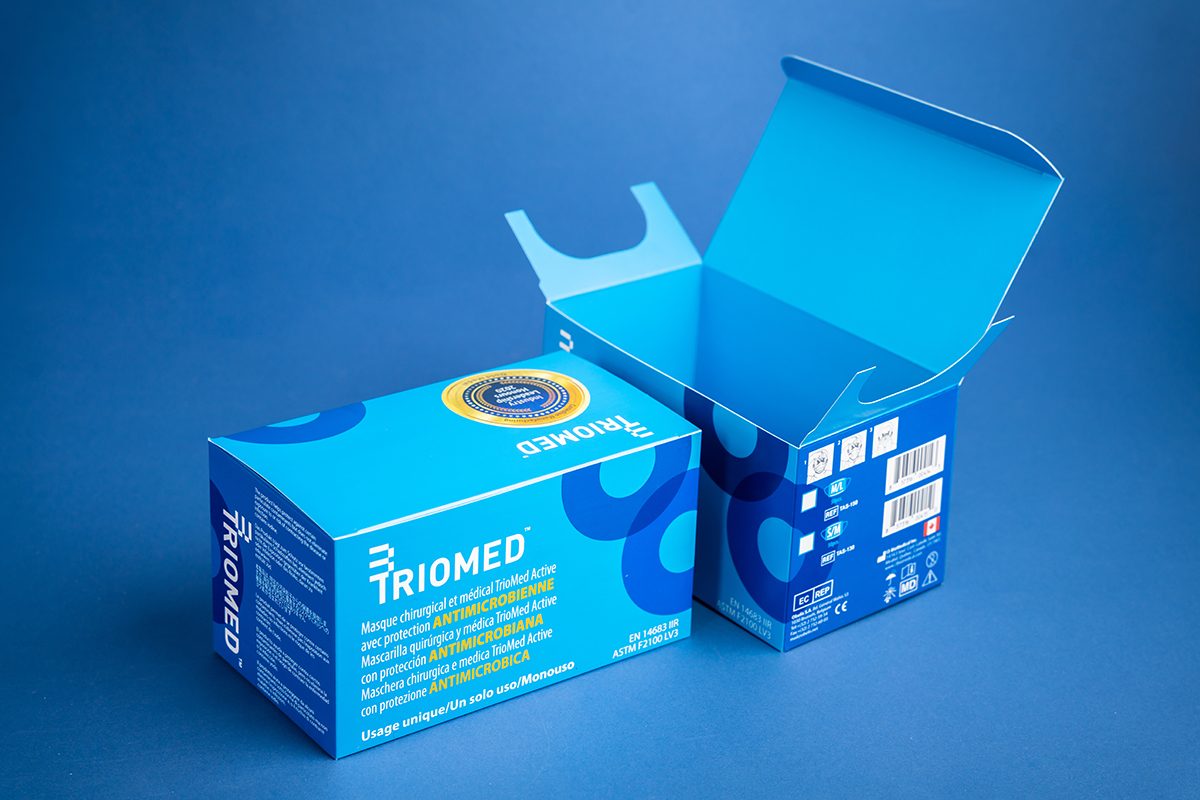
Standout Graphics and Branding - Utilize vibrant colors, eye-catching graphics, and brand logos to make the packaging visually appealing and easily recognizable.
High-Quality Printing - Utilize high-quality printing techniques to ensure sharp images, vibrant colors, and clear text on the custom boxes. This enhances the packaging's visual appeal and readability.
Customized Finishes and embellishments - Choose custom finishes like matte, gloss, or soft-touch lamination to add texture and enhance the tactile experience of handling the packaging. Add foil stamping or embossing to highlight specific elements on the packaging.

Product Visibility - Use transparent windows or cutouts on the packaging to let consumers see the product inside.
Multi-Packs and Bundles - Offer special deals or discounts for multi-packs or bundled products, and display them together to encourage customers to purchase more items.
Interactive QR Codes - customers can scan them with their smartphones to access additional product information, videos, or promotions.

Point-of-Sale Displays – Develop countertops or freestanding displays that prominently showcase the product and its packaging.
Ensuring Quality: Best practices for medical packaging
Best practices for medical packaging involve a combination of design, material selection, manufacturing processes, and quality control measures.
Regulatory Compliance - Adherence to regulatory requirements is paramount. Medical packaging must meet the relevant standards and guidelines set by health authorities.
Packaging Design - Ensure that the packaging design is user-friendly and meets the needs of healthcare professionals and patients. Consider ease of opening, clear instructions, and ergonomic design. Use tamper-evident features to ensure that medical products remain protected and uncontaminated.

Labeling Accuracy - Accurate and clear labeling is essential to provide essential information about the medical product, including its contents, instructions for use, expiration date, lot number, and any warnings or precautions.
Stability and Shelf Life - Conduct stability testing to determine the appropriate shelf life of medical products and package them in a manner that maintains their stability over time.
Environmental Considerations - Use environmentally friendly packaging materials and optimize package size to minimize waste generation.
Compatibility Testing - Perform compatibility testing between the medical product and its packaging to ensure no adverse interactions could compromise the product's quality or safety.
Supply Chain Considerations - Consider the entire supply chain, from manufacturing to distribution and storage, to ensure that the packaging can withstand various transportation and storage conditions.
Risk Management - Implement risk management practices to identify and mitigate potential risks associated with medical packaging failures.
Sustainability Practices: Eco-friendly approaches for pharmaceutical packaging
Eco-friendly approaches for pharmaceutical packaging focus on reducing the environmental impact of packaging materials and processes used in the pharmaceutical industry. Here are some eco-friendly strategies for pharmaceutical packaging:
Sustainable Materials - Opt for packaging materials that are renewable, recyclable, or biodegradable. Use paperboard, cardboard, or other plant-based materials for secondary packaging and inserts. Check out this informative article on What Is Sustainable Packaging Design.
Recycled Content - Incorporate recycled materials into packaging whenever possible. Using post-consumer recycled content helps reduce the demand for virgin materials and lowers the overall environmental footprint.
Eco-Friendly Inks and Coatings -Use environmentally friendly printing inks and coatings free from harmful chemicals.
Sustainable Sourcing - Source packaging materials from suppliers with sustainable practices and certifications, such as Forest Stewardship Council (FSC) for paper products.

Recycling and Disposal Guidance - Provide clear instructions to consumers on adequately recycling or disposing of the packaging to promote responsible waste management.
Collaborative Initiatives - Partner with packaging suppliers, recycling organizations, and industry associations to develop and support eco-friendly packaging initiatives.
In summary, pharmaceutical packaging is critical in product protection, safety, compliance, and marketing. Custom boxes for pharmaceutical products ensures that pharmaceutical products are well-protected and aligned with the brand's identity and sustainability goals.