These food packaging guidelines can help everybody who is involved in creating food packaging to make sure that the packaging complies with the regulations.
When companies think about food packaging, it is usually focused on making it attractive and enticing the customers to buy the product. However, if you want to reach the customers, you need to ensure that the packaging complies with all the existing laws and regulations on your targeted market. This article offers a general overview of the food labeling regulations in the USA and Canada and the main differences between regulations in these two countries.
However, you should use these food packaging guidelines for informational purposes only, as some foods are more heavily regulated than others, and regulations are subject to change. We will discuss FDA food labeling requirements for the US market and the Health Canada food labeling regulations for the Canadian market.
Food Packaging Principal Display Panel
Principal display panel (PDP) is a surface that is most visible to the consumer on the shelf or at the time of purchase. The area panel on the immediate right of the PDP is the Informational panel.

Required Label Information for Most Food Packaging in the USA
There is certain required label information that must be printed on the two panels mentioned above. Common name and net quantity must be printed on the PDP panel and the Informational panel.
Every food packaging must include:
- Common name
- Net quantity
- Information about manufacturer, packer and/or distributor
- Ingredients
- Nutrition facts
- Allergens
The Information Panel must not contain any other information or design elements not required by the FDA.
Required Label Information for Most Food Packaging in Canada
Canadian food labeling regulations also require the name of the product and net quantity to be printed on the PDP. However, other required information can be placed anywhere on the packaging and they include:
- Common name
- Net quantity
- Information about primary business or dealer
- Storage instructions (If food is not to be stored at room temperature)
- Durable life date
- Ingredients
- Nutrition Facts Table
- Allergen labeling

Main Differences in the US and Canadian Food Labeling Regulations
Canadian regulations require bilingual information and a “best before” date which is not mandatory in the USA.

Also, note that starting January 1, 2022. the National Bioengineered Food Disclosure Standard is effective in the USA. There is no equivalent standard in Canada. This standard defines bioengineered food as food containing modified genetic material that could not be obtained through conventional breeding or found in nature. There is a List of bioengineered foods for which disclosure is necessary.
There are two exemptions:
- Food served in a restaurant or similar establishments and
- Very small food manufacturers.
Several available disclosure options are:
- Text,
- Symbol,
- Electronic or digital link.

Different Elements of Food Packaging Guidelines Explained
1. Common Name
Common name (“ice cream”, “sandwich”) must be visibly displayed with the format of the food (if there are different formats available on the market).
2. Net Quantity
Net quantity is the amount of food in the food packaging. It must be given in weight, measure, or count. Generally, solid foods are measured by weight and liquid foods in fluid ounces.

Main Differences Regarding the Net Quantity
In the USA, net quantity has to be printed in both US Customary and metric measurements.
In Canada, the net quantity must be expressed in metric units in both French and English. It is permitted to use imperial units, but it is not required.
The USA have no rounding requirements but must not exceed two decimal places. The net quantity must be rounded to two figures in Canada if it is less than 100 units, or three if it is more than 100 units.
Keep This in Mind Regarding the Net Quantity:
- Frozen foods should be measured frozen.
- Packaging of any kind should not be included when determining the net quantity
- The type size is determined by measuring the lowercase “o”.
- Minimal type size depends on the total area of the PDP
3. Information About Manufacturer, Packer and/or Distributor
The information on food packaging must specify the name and address of the manufacturer or distributor. Both countries’ requirements regarding the manufacturer identity statement are more or less the same, although Canada has more specific wording requirements for the products manufactured in other countries. Even though FDA does not require country of origin information to be on the label, note that USA Customs and Border Protection does. An exception to this rule is when the product undergoes a “substantial transformation” in the USA.
4. Ingredients List
It is important to note that it is not possible that the same Nutritional Facts panel can satisfy both FDA food labeling requirements and Health Canada food labeling regulations.
The US ingredients list has to be located on the PDP or Information panel, together with the information about the manufacturer or distributor. In Canada, it can be located anywhere on the packaging, except for the bottom panel for most products. Even the bottom panel is acceptable, although only for very specific types of packaging (like ornamental). Since there are many differences between the two countries and many rules regarding ingredients, we will not go into details in these food packaging guidelines.
However, we want to make sure that you are aware that those differences exist.
Also, note that in Canada, there have been food labeling changes regarding the list of ingredients and nutritional information for which CFIA will verify compliance as of December 15, 2022.
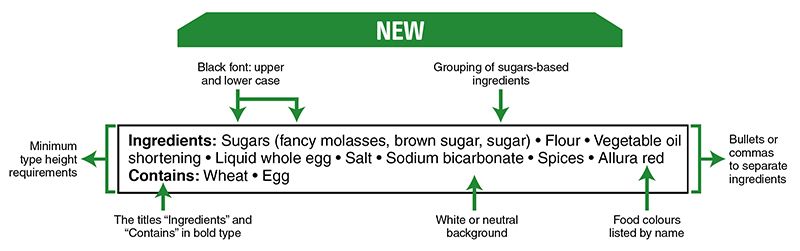
There are changes to sweetener information on food labels. The changes concern foods that contain the following high-intensity sweeteners approved by Health Canada for use in Canada:
- neotame
- sucralose
- aspartame
- acesulfame-potassium

5. Nutrition Facts
In the USA, you should put nutritional information on the PDP or information panel whenever possible. Nutritional labels must be printed in all black or one colour type on a white or neutral contrasting background and must be readable. There have been changes in the FDA food labeling requirements regarding nutrition facts, and the transition period has ended on January 1, 2021.
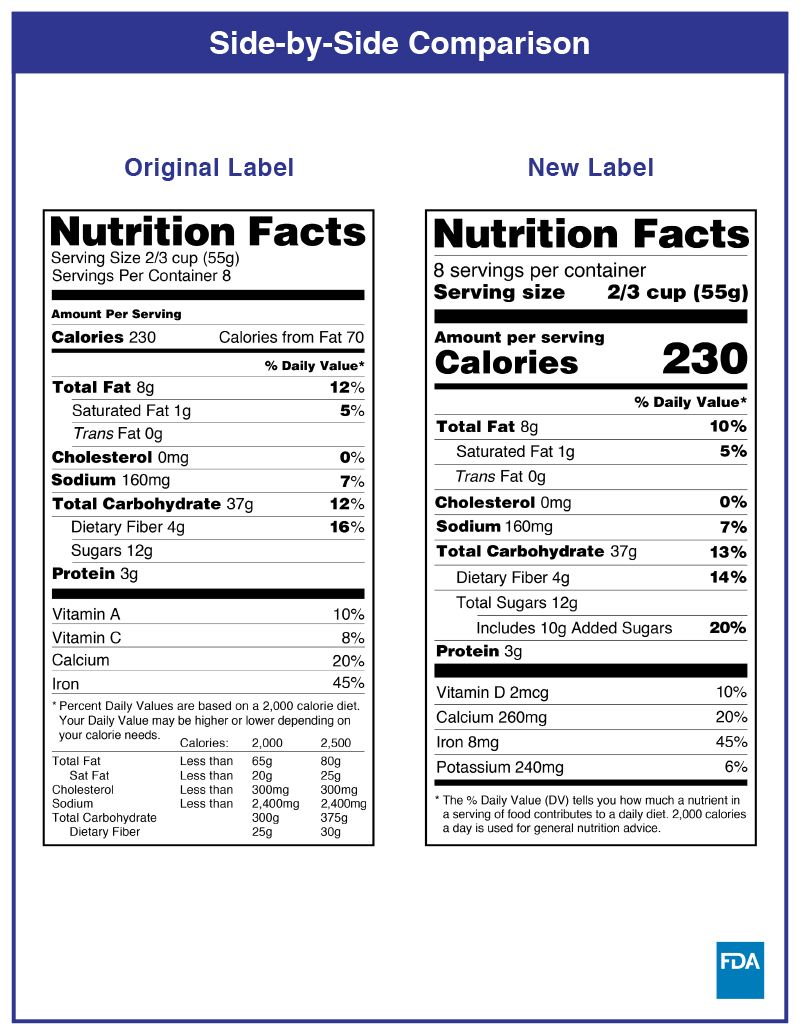
Health Canada closely regulates the size and appearance of the NFT. Multiple formats can be used, and the choice depends on the precise calculation of the Available Display Surface.

There are changes that CFIA will verify compliance for as of December 15, 2022.
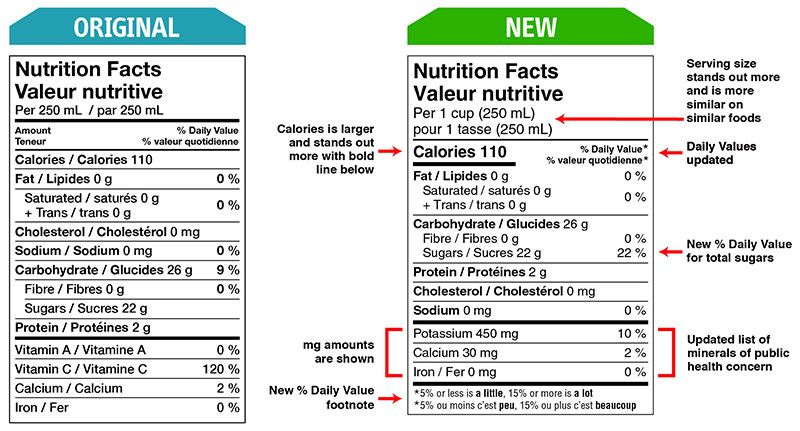
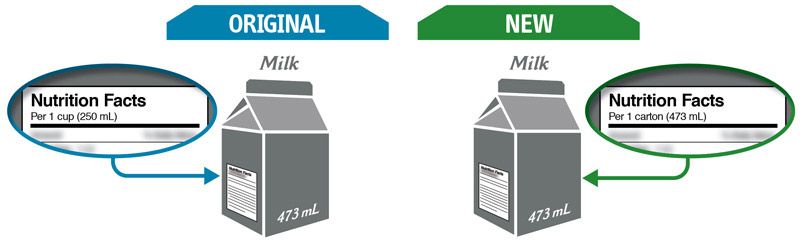
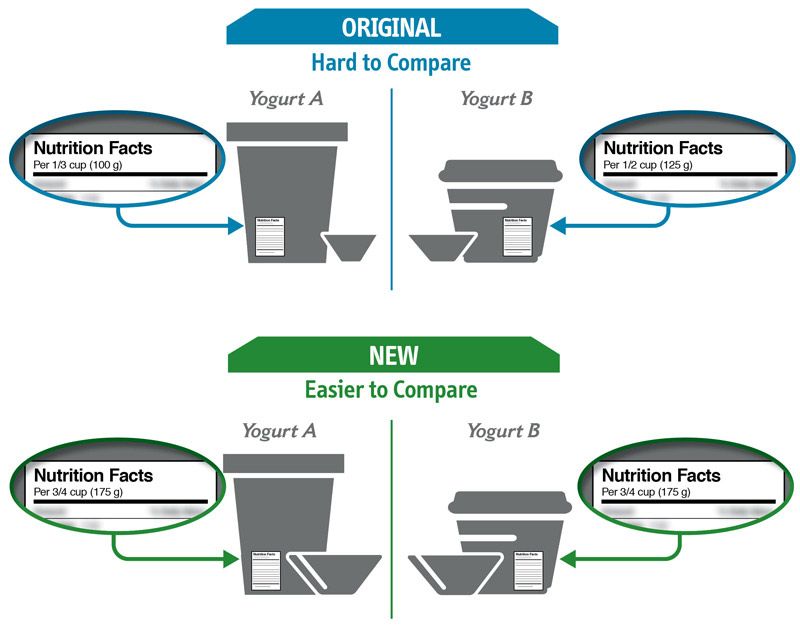
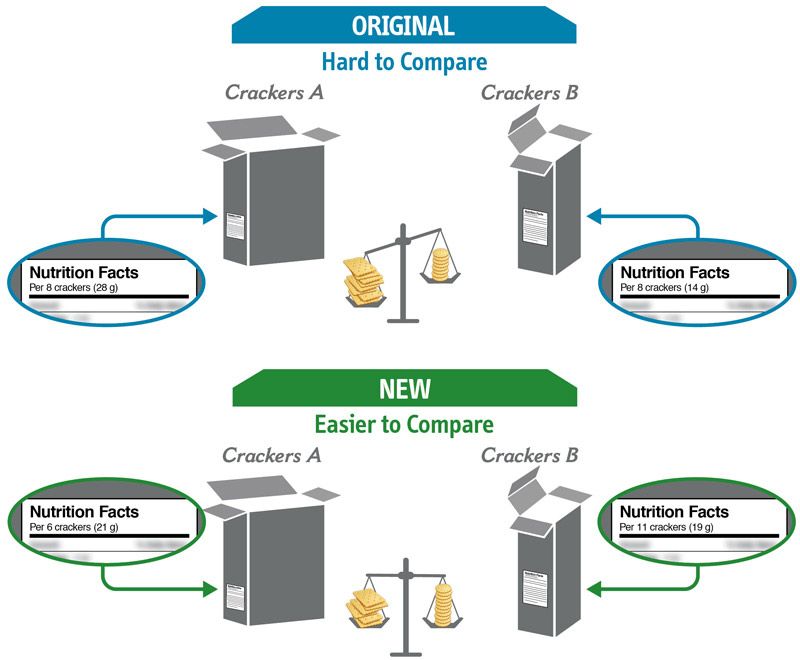
Some changes in serving size information are made to make it easier to compare products.
A % daily value has been included for total sugars.
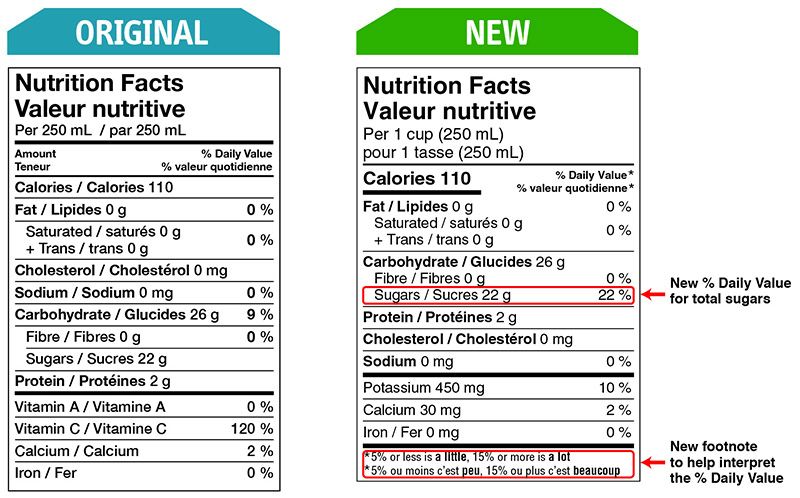
A front-of-package nutrition symbol is required on foods that are high in one or more of these nutrients:
- sodium
- sugars
- saturated fat
The food industry has been given until January 1, 2026 to make this change.

6. Allergen Labeling
You can choose one of the two acceptable ways to list allergens. Either you can declare the allergen in parenthesis or add a “Contains” statement at the end of the ingredients list. This is the same both for FDA food labeling requirements and Health Canada regulations.

7. Durable Life Date
Canadian food labeling regulations require “best before” and expiry dates for products with a shelf life shorter than 90 days. FDA food labeling requirements do not require any kind of durable life labeling for products, except for infant formula. However, the FDA strongly supports efforts to include a “best if used by” phrase to help reduce unnecessary food waste.
We hope that these food packaging guidelines will help you know what to pay close attention to when creating food packaging for your products. There are certain products that are exempted from these regulations, so make sure that you have all the relevant information regarding your product before designing and printing food packaging.












